
Histone H3 lysine 9 trimethylation (H3K9me3) plays critical roles in maintaining heterochromatin structure, gene silencing, and DNA damage repair. Dysregulation of H3K9me3 can lead to genomic instability and cancer development. The establishment of H3K9me3 depends on the reader protein HP1 recognizing pre-existing modifications and recruiting the writer methyltransferase SUV39H1, which methylates adjacent newly incorporated histones to form a "read-write" positive feedback loop. This process requires strict regulation to prevent excessive H3K9me3 deposition and aberrant heterochromatin formation. However, mechanisms restricting this positive feedback to maintain H3K9me3 homeostasis in higher organisms remain unclear.
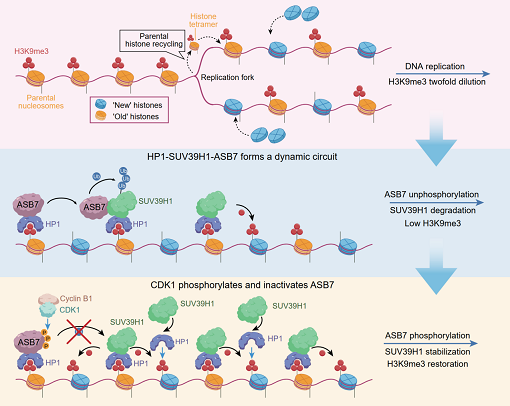
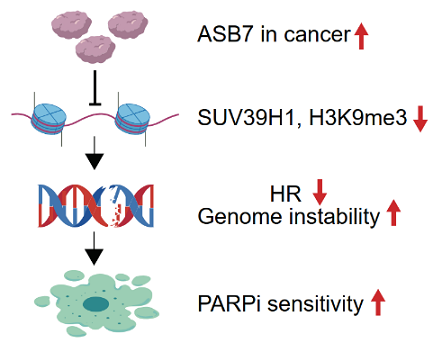
H3K9me3 modification occurs at DNA double-strand break (DSB) sites to activate repair pathways. ASB7 amplification in multiple tumor types reduces H3K9me3 levels at DSB sites, impairing homologous recombination repair (HRR). Cellular and animal experiments demonstrate that high ASB7 expression enhances tumor sensitivity to PARP inhibitors. These findings suggest patients with ASB7-amplified tumors may represent a potential beneficiary population for PARP inhibitor therapy.
Zhou Liwen, Associate Professor from the Experimental Research Department of Sun Yat-sen University Cancer Center, Chen Zhenxuan, a Master's candidate, and Zou Yezi, a Ph.D. candidate from Sun Yat-sen University School of Medicine, are the co-first authors of the paper. Prof. Kang Tiebang and Associate Prof. Wu Yuanzhong, from the Experimental Research Department of Sun Yat-sen University Cancer Center, are the corresponding authors.
Written by: Prof. Wu Yuanzhong, Experimental Research Department
Original Link: https://www.science.org/doi/10.1126/science.adq7408
Copyright:Sun Yat-sen University Cancer Center Designed by Wanhu.