A global multicenter, randomized, double blind, phase III clinical study (RATIONALE-305 study) led by Prof. Rui-hua Xu from Sun Yat-sen University Cancer Center (SYSUCC) was published in the BMJ, one of the four world-renowned comprehensive medical journals. The study confirms that the addition of the anti-PD-1 antibody tislelizumab to chemotherapy can significantly prolong the overall survival of advanced gastric cancer patients compared to placebo combined chemotherapy. This provides a new primary treatment option for patients with advanced gastric or gastro-oesophageal junction adenocarcinoma.

Gastric cancer is one of the most common types of cancer and one of the leading causes of cancer related deaths, with both the incidence and mortality rate ranking fifth among all cancer types globally. With heavy cancer burden and high heterogeneity, the 5-year survival rate in advanced gastric cancer patients is lower than 10%. Therefore, developing effective treatment options for advanced gastric cancer patients is of vital importance.
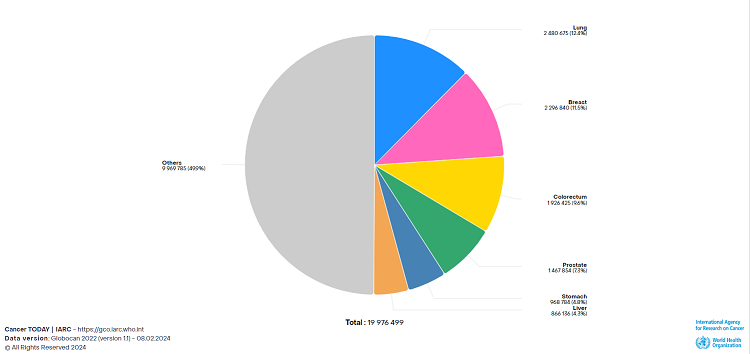
Adopted from Cancer Today, IARC, WHO
Study design, interventions and main outcome measuresSpecifically engineered to minimise Fcγ receptor binding on macrophages, tislelizumab is an anti-PD-1 monoclonal antibody with high affinity. The RATIONALE-305 study evaluated the efficacy and safety of tislelizumab plus chemotherapy compared with placebo plus chemotherapy as primary treatment for locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma.
The study was carried out in 146 medical centers across Asia, Europe, and North America, between 13 December 2018 and 28 February 2023. Overall, 997 patients with previously untreated HER2 negative locally advanced unresectable or metastatic gastric or gastrooesophageal junction adenocarcinoma were included in the study cohort.
Patients were randomly (1:1) assigned to receive either tislelizumab or placebo in combination with chemotherapy (investigator’s choice of oxaliplatin and capecitabine, or cisplatin and 5-fluorouracil) and stratified by region, PD-L1 expression, presence or absence of peritoneal metastases, and investigator’s choice of chemotherapy. Treatment continued until disease progression or unacceptable toxicity.
The primary endpoint was overall survival, both in patients with a PD-L1 tumour area positivity (TAP) score of ≥5% and in all randomised patients. Safety was assessed in all those who received at least one dose of study treatment.
Results and conclusions
Tislelizumab plus chemotherapy showed statistically significant improvements in overall survival versus placebo plus chemotherapy in patients with a PD-L1 TAP score of ≥5% (median 17.2 months v 12.6 months; hazard ratio 0.74 (95% CI: 0.59 to 0.94); P=0.006 (interim analysis)) and in all randomized patients (median 15.0 months v 12.9 months; hazard ratio 0.80 (0.70 to 0.92); P=0.001 (final analysis)). Grade 3 or worse treatment related adverse events were observed in 54% of patients in the tislelizumab plus chemotherapy arm versus 50% in the placebo plus chemotherapy arm.

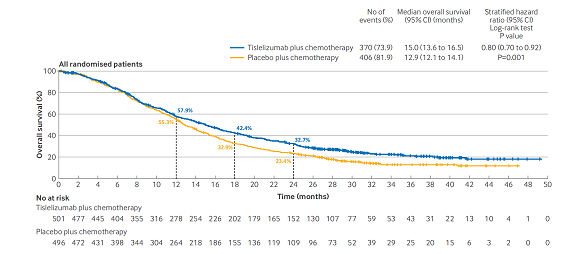
Kaplan-Meier plots of overall survival in population with PD-L1 TAP scores of ≥5% and in all randomised patients
Primary treatment of advanced gastric or gastrooesophageal junction adenocarcinoma with tislelizumab plus chemotherapy provided significant and clinically meaningful overall survival benefit versus placebo plus chemotherapy in patients with a PD-L1 TAP score of ≥5%, and in all randomized patients. The safety profile of tislelizumab in combination with chemotherapy was manageable and acceptable. The study results suggest that tislelizumab plus chemotherapy represents a potential new primary treatment option for patients with advanced gastric or gastro-oesophageal junction adenocarcinoma.
Led by Prof. Rui-hua Xu from Sun Yat-sen University Cancer Center, the RATIONALE-305 study was conducted in 146 medical centers across Asia, Europe, and North America. This study exemplifies SYSUCC's success in promoting cancer treatment innovation through international research collaboration.
Link to the original article: https://www.bmj.com/content/385/bmj-2023-078876
Written by: Liao Shuang, Zhai Huiwen
International Office,
Sun Yat-sen University Cancer Center



